Spadefoots!
An overview of their ecology, evolution, development, genetics, and behavior
What is a spadefoot?
The first thing you need to know about spadefoots toads (hereafter, “spadefoots”) is that they’re not actually toads –– they’re frogs. True toads are restricted to the genus Bufo (Anaxyrus). Spadefoots get their name from the keratinized knob (or “spade”) on their hind feet that they use for digging (Figure 1).

Figure 1. Spadefoots, such as this Mexican spadefoot (Spea multiplicata), are equipped with numerous adaptations for living in dry environments, including their keratinized “spade” (arrow), which enables them to burrow underground to escape desiccation (inset). This male is calling to attract a mate. Photo taken near Portal, Arizona.
Where do we find spadefoots?
Spadefoots are found in both the Old World and the New World. The European spadefoots (family Pelobatidae) consist of one genus (Pelobates) containing four species that are native to Europe, northwestern Africa, and western Asia. The American spadefoots (family Scaphiopodidae) consist of two genera (Scaphiopus [3 species] and Spea [4 species]), which occur throughout North America (Figure 2).
Two species––Scaphiopus holbrookii and Sc. hurterii––are native to eastern and central North America (Figures 2, 3), whereas the remaining five species––Sc. couchii, Spea multiplicata, Sp. bombifrons, Sp. hammondii, and Sp. intermontana––occur in arid western North America (Figures 2, 4). Indeed, spadefoots are unusual among amphibians in that they’ve found a way to live in deserts.
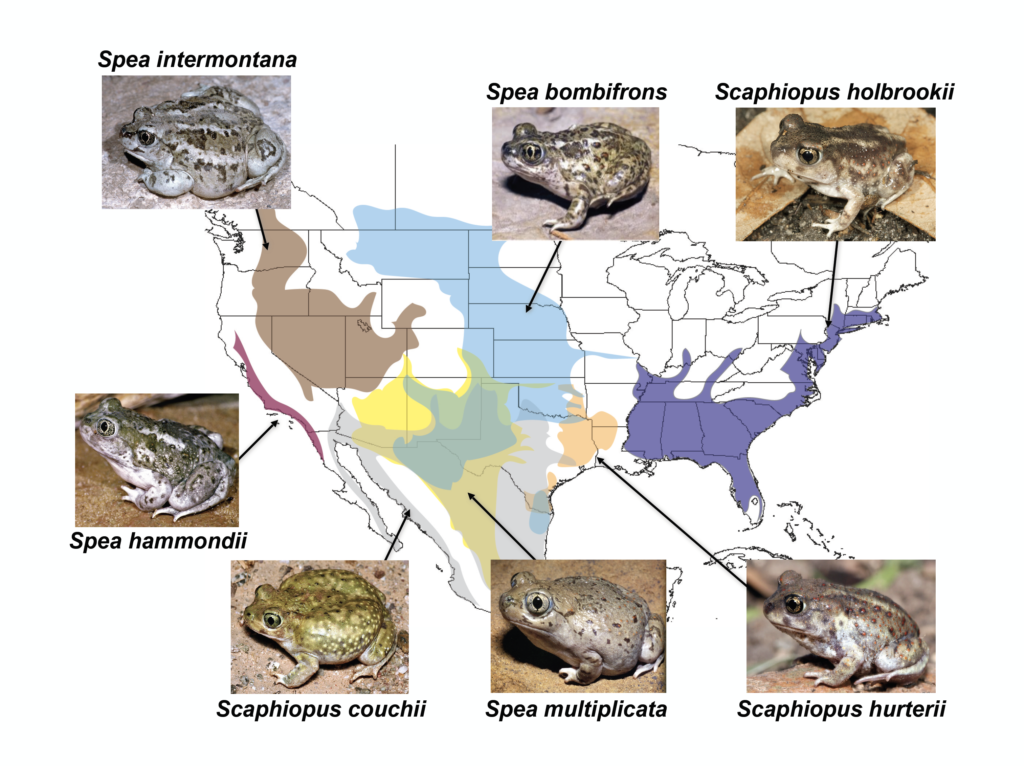
Figure 2. Diversity and distributions of American spadefoots.
What does it look like where spadefoots live?

Figure 3. An ephemeral pond near Hoffman, North Carolina, with tadpoles (inset) of Eastern spadefoots (Scaphiopus holbrookii).

Figure 4. A spadefoot breeding pond in southeastern Arizona, which filled hours before this photo was taken (the storm looms over the Chiricahua Mountains). Spadefoots emerge from their underground burrows for only a few weeks each year to feed and breed in these temporary, rain-filled ponds. Spea multiplicata and Sc. couchii breed in this pond.
How can spadefoots live in such extreme environments?
Spadefoots have evolved numerous adaptations to cope with arid (and often unpredictable) environments. These adaptations include prolonged dormancy, rapid growth and development, phenotypic plasticity, and adaptive mating between species (hybridization).
Adaptation No. 1
Prolonged Dormancy
Spadefoots are behaviorally and physiologically equipped to survive in arid environments. Adults escape dry conditions by burrowing underground using their spade (see Figure 1). Some species burrow over a half a meter deep!
Once underground, spadefoots can remain dormant for a year or longer. During this prolonged dormancy, they reduce their metabolism and conserve water by storing waste as urea and by encasing themselves in multiple protective layers of unshed skin.
They emerge from these burrows following warm rains (Figures 5, 6), which they may detect by hearing the low-frequency sound (< 100 Hz) that characterizes heavy rain striking the surface of the ground above them.

Figure 5. Typical habitat of Sc. couchii, Sp. bombifrons, Sp. multiplicata in southeastern Arizona. Spadefoots live underground for much of the year in a prolonged dormancy, emerging for only a few weeks following warm rains (such as this storm) to breed and feed.

Figure 6. Scaphiopus holbrookii emerging from its burrow following a rainstorm.
Adaptation No. 2
Rapid Growth and Development
Regardless of whether or not they live in the desert, spadefoots breed in temporary, rain-filled ponds (see Figures 3, 4). Although most frogs and toads could not reproduce in such highly ephemeral environments, spadefoot tadpoles can survive in them by developing rapidly––in some cases metamorphosing in eight days post-hatching (Figure 7).
Furthermore, spadefoot tadpoles develop lungs relatively early in life. Consequently, if their pond dries, they can remain alive on dry land for days (Figure 8), thereby increasing their chances of surviving until another rainstorm refills their pond.

Figure 7. Spadefoot tadpoles exhibit rapid, and adaptively flexible, larval development. Here, a Spea multiplicata has developed its limbs and thereby escapes its rapidly drying pond (photo near Portal, Arizona).

Figure 8. Because they develop lungs early, Spea tadpoles can survive on land as tadpoles for days. These Spea multiplicata tadpoles (encased in mud) are still alive. However, they are vulnerable to predators, such as these tiger beetles.
Adaptation No. 3
Phenotypic Plasticity
Spadefoots also exhibit multiple forms of phenotypic plasticity that allows them to thrive in environments (such as deserts) where rainfall is highly variable. Specifically, in response to changing water levels in their pond, spadefoot tadpoles can either speed up or slow down their development.
Moreover, whereas most frog and toad tadpoles are omnivores and exhibit traits adapted for feeding on detritus and plankton, tadpoles of the genus Spea can develop into an alternative––and more rapidly developing––carnivore morph, which exhibits enlarged jaw muscles and mouthparts for capturing and consuming large animal prey, such as fairy shrimp and other tadpoles (Figure 9).
The carnivore morph has an additional advantage in a rapidly drying pond: by eating its competitors, it can reduce competition and further enhance growth and development (Figure 10). However, when engaging in cannibalism, carnivores can distinguish between kin and nonkin, preferentially cannibalizing the latter.

Figure 9. Spea produce alternative, environmentally induced, tadpole morphs: a slower developing omnivore morph (left) and a more rapidly developing carnivore morph (right), which is induced by, and specializes on, animal prey, such as fairy shrimp (center).

Figure 10. Spea carnivore-morph tadpoles are highly cannibalistic. Here, a carnivore Sp. multiplicata eats an omnivore.
Adaptation No. 4
Adaptive Mating Between Species
Finally, as adults, at least one species of spadefoot exhibits remarkable behavioral plasticity. Such plasticity appears to have enabled this species to invade desert habitats.
In particular, when breeding in shallow, rapidly drying ponds, Sp. bombifrons females often switch from mating with their own species (Figure 11) to preferring males of Sp. multiplicata (Figure 12). In the desert southwest, these two species often breed in the same ponds, and Sp. multiplicata tadpoles develop faster than Sp. bombifrons tadpoles.
Because the resulting Sp. bombifrons x Sp. multiplicata hybrid tadpoles (which are viable) develop faster than pure-Sp. bombifrons tadpoles, they are more likely to escape a drying pond. As long as some of these hybrid offspring mature and ultimately reproduce successfully (as genetic evidence suggests that they do), this form of behavioral plasticity can be an adaptive––albeit extreme––strategy for dealing with dry environments.

Figure 11. A mated pair (male on back of female) of Plains spadefoots, Spea bombifrons.

Figure 12. A male Mexican spadefoot, Sp. multiplicata (left), mated with a female Plains spadefoot, Spea bombifrons (right).
How do we track adaptations?
To help begin to identify the genetic bases of spadefoots’ unique adaptations, we recently sequenced the genome of Spea multiplicata.
One discovery that we’ve already made is that Sp. multiplicata possesses one of the smallest amphibian genomes known (Figure 13A). Moreover, compared to the genomes of other sequenced frog and toad species, the genome of Sp. multiplicata contains less repetitive DNA (DNA sequences that are repeated in the genome and that do not code for protein; Figure 13B). Additionally, relative to these other sequenced frog and toad species, Sp. multiplicata also has smaller genes (Figure 13C). The fact that the different species differ in gene length, but not in transcript length (see Figure 13C), suggests that Sp. multiplicata has smaller genes because some non-coding sequences have been purged from their genes.
Such a reduction in genome and gene size makes sense. As noted above, Sp. multiplicata experiences strong selection to develop rapidly: one way to speed development is to have less DNA to replicate!
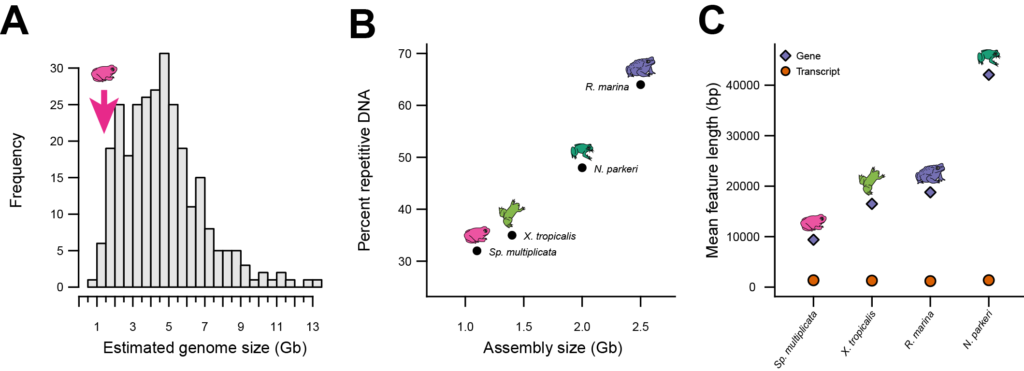
Figure 13. Factors contributing to differences in genome size among frogs and toads. A Estimated genome sizes of 284 species of anurans; arrow: genome size of Spea. B Genome (assembly) size and C gene and transcript lengths of Sp. multiplicata compared to three other species whose genomes have been sequenced. Figure from Seidl et al. (2019).
Cool! How can I learn more?
Below are some videos and a list of scientific papers for you to learn more about these remarkable creatures.
Sp. multiplicata male calling
Sp. multiplicata male calling and then being chosen by a female
Sp. bombifrons male calling
Sc. couchii male calling
Sp. multiplicata tadpoles in the wild
Sp. multiplicata carnivores and omnivores from a single clutch of eggs, showing difference between morphs in behavior, even among siblings
Here’s a short (< 5 min.) video on our spadefoot research:
From the National Science Foundations’s Science Nation series.
Here are some publications on spadefoots:
Bragg, A. N. 1965. Gnomes of the night: the spadefoot toads. University of Pennsylvania Press, Philadelphia, PA.
PDF Chen, C. and K. S. Pfennig. 2020. Female toads engaging in adaptive hybridization prefer high-quality heterospecifics as mates. Science 367:1377.
Denver, R. J., N. Mirhadi, and M. Phillips. 1998. Adaptive plasticity in amphibian metamorphosis: response of Scaphiopus hammondii tadpoles to habitat desiccation. Ecology 79:1859-1872.
Dimmitt, M. A. & Ruibal, R. 1980. Environmental correlates of emergence in spadefoot toads (Scaphiopus). Journal of Herpetology 14: 21-29.
Gomez-Mestre, I. and D. R. Buchholz. 2006. Developmental plasticity mirrors differences among taxa in spadefoot toads linking plasticity and diversity. Proceedings of the National Academy of Sciences, U.S.A. 103:19021-19026.
PDF Kelly, P. W., D. W. Pfennig, S. de la Serna Buzón, and K. S. Pfennig. 2019. Male sexual signals predict phenotypic plasticity in offspring: implications for the evolution of plasticity and local adaptation. Philos. Trans. R. Soc. B-Biol. Sci. 374:20180179.
PDF Ledón-Rettig, C. C. and D. W. Pfennig. 2011. Emerging model systems in eco-evo-devo: the environmentally responsive spadefoot toad. Evolution and Development 13:391-400.
PDF Levis, N. A., A. Isdaner, and D. W. Pfennig. 2018. Morphological novelty emerges from pre-existing phenotypic plasticity. Nature Ecology & Evolution 2:1289–1297.
PDF Levis, N. A., R. A. Martin, K. A. O’Donnell, and D. W. Pfennig. 2017. Intraspecific adaptive radiation: competition, ecological opportunity, and phenotypic diversification within species. Evolution 71:2496–2509.
PDF Martin, R. A. and D. W. Pfennig. 2009. Disruptive selection in natural populations: the roles of ecological specialization and resource competition. Am. Nat. 174:268-281.
PDF Pfennig, K. S. and Pfennig, D. W. 2020. Dead spadefoot tadpoles adaptively modify development in future generations: a novel form of nongenetic inheritance? Copeia 108: 116-121.
PDF Pfennig, D. W. 1990. The adaptive significance of an environmentally-cued developmental switch in an anuran tadpole. Oecologia 85:101-107.
PDF Pfennig, D. W. 1992. Polyphenism in spadefoot toads as a locally adjusted evolutionarily stable strategy. Evolution 46:1408-1420.
PDF Pfennig, D. W. and P. J. Murphy. 2002. How fluctuating competition and phenotypic plasticity mediate species divergence. Evolution 56:1217-1228.
PDF Pfennig, D. W., H. K. Reeve, and P. W. Sherman. 1993. Kin recognition and cannibalism in spadefoot toad tadpoles. Anim. Behav. 46:87-94.
PDF Pfennig, K. S. 2000. Female spadefoot toads compromise on mate quality to ensure conspecific matings. Behavioral Ecology 11:220-227.
PDF Pfennig, K. S. 2007. Facultative mate choice drives adaptive hybridization. Science 318:965-967.
PDF Pfennig, K. S. & Rice, A. M. 2014. Reinforcement generates reproductive isolation between neighbouring populations of spadefoot toads. Proceedings of the Royal Society B: Biological Sciences 281: 20140949.
PDF Pfennig, K. S. and R. C. Tinsley. 2002. Different mate preferences by parasitized and unparasitized females potentially reduces sexual selection. Journal of Evolutionary Biology 15:399-406.
PDF Pierce, A. A., Gutierrez, R., Rice, A. M. & Pfennig, K. S. 2017. Genetic variation during range expansion: effects of habitat novelty and hybridization. Proceedings of the Royal Society B: Biological Sciences 284: 20170007.
Pomeroy, L. V. 1981. Developmental polymorphism in the tadpoles of the spadefoot toad Scaphiopus multiplicatus. University of California, Riverside, CA.
Ruibal, R., L. Tevis, and V. Roig. 1969. The terrestrial ecology of the spadefoot toad Scaphiopus hammondii. Copeia 1969:571-584.
PDF Seidl, F., N. A. Levis, R. Schell, D. W. Pfennig, K. S. Pfennig, and I. M. Ehrenreich. 2019. Genome of Spea multiplicata, a rapidly developing, phenotypically plastic, and desert-adapted spadefoot toad. G3: Genes, Genomes and Genetics 3909–3919.
Seymour, R. S. 1973. Energy metabolism of dormant spadefoot toads (Scaphiopus). Copeia 1973:435–445.
Vásquez, T. & Pfennig, K. S. 2007. Looking on the bright side: females prefer coloration indicative of male size and condition in the sexually dichromatic spadefoot toad, Scaphiopus couchii. Behavioral Ecology and Sociobiology 62: 127-135.
Zeng, C., I. Gomez-Mestre, and J. J. Wiens. 2014. Evolution of rapid development in spadefoot toads is unrelated to arid environments. PLoS ONE 9:e96637.
