Our Research
Phenotypic Plasticity, Character Displacement, and Mimicry
Arrival of the Fittest: Phenotypic Plasticity
A recent, controversial idea holds that phenotypic plasticity plays a crucial role in both innovation and diversification.
OVERVIEW
A central goal of evolutionary biology is to explain the origin and diversification of complex traits. A recent, controversial idea holds that phenotypic plasticity––the ability of an individual to modify its traits in direct response to changes in its environment––plays a crucial role in both innovation and diversification. According to this “plasticity-led (or plasticity-first) hypothesis” of adaptive evolution, when selection acts on quantitative genetic variation regulating the expression of an initially environmentally induced trait, it can promote the evolution of either increased or decreased plasticity (an evolutionary process dubbed “genetic accommodation”). If the affected trait evolves decreased plasticity to the point of becoming constitutively expressed, “genetic assimilation” occurs. The outcome of genetic assimilation is a novel, canalized trait.
Although lab studies have demonstrated that genetic accommodation/assimilation can occur, whether it actually has occurred and contributed to the evolution of any complex, novel traits in natural populations remains unclear. We have been using spadefoot toads as model systems to test critical predictions of the plasticity-led evolution hypothesis for the evolutionary origins of novelty. We’ve also been using this system to evaluate whether and how phenotypic plasticity promotes rapid divergence between populations and, ultimately, reproductive isolation. Finally, we’ve been studying: 1) the degree to which plasticity fosters biodiversity indirectly by promoting species persistence when confronted by rapid environmental change (such as the presence of a novel competitor), and 2) the unique role of behavioral plasticity in instigating evolutionary innovation.
Recent representative papers:
PDF Pfennig, D. W. 2022. Evolution and the flexible organism. American Scientist 110: 94– 101.
PDF Levis, N. A., Kelly, P. W., Harmon, E. A., Ehrenreich. I. M., McKay, D. J., and Pfennig, D. W. 2021. Transcriptomic bases of a polyphenism. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 336:482–485.
PDF Levis, N. A., Reed, E. M. X., Pfennig, D. W., Burford Reiskind, M. O. 2020. Identification of candidate loci for adaptive phenotypic plasticity in natural populations of spadefoot toads. Ecology and Evolution 10: 8976–8988.
PDF Levis, N. A., Isdaner, A., and Pfennig, D. W. 2018. Morphological novelty emerges from pre-existing phenotypic plasticity. Nature Ecology and Evolution 2: 1289–1297.
PDF Levis, N. and Pfennig, D. W. 2016. Evaluating ‘plasticity-first’ evolution in nature: key criteria and empirical approaches. Trends in Ecology and Evolution 31: 563-574.
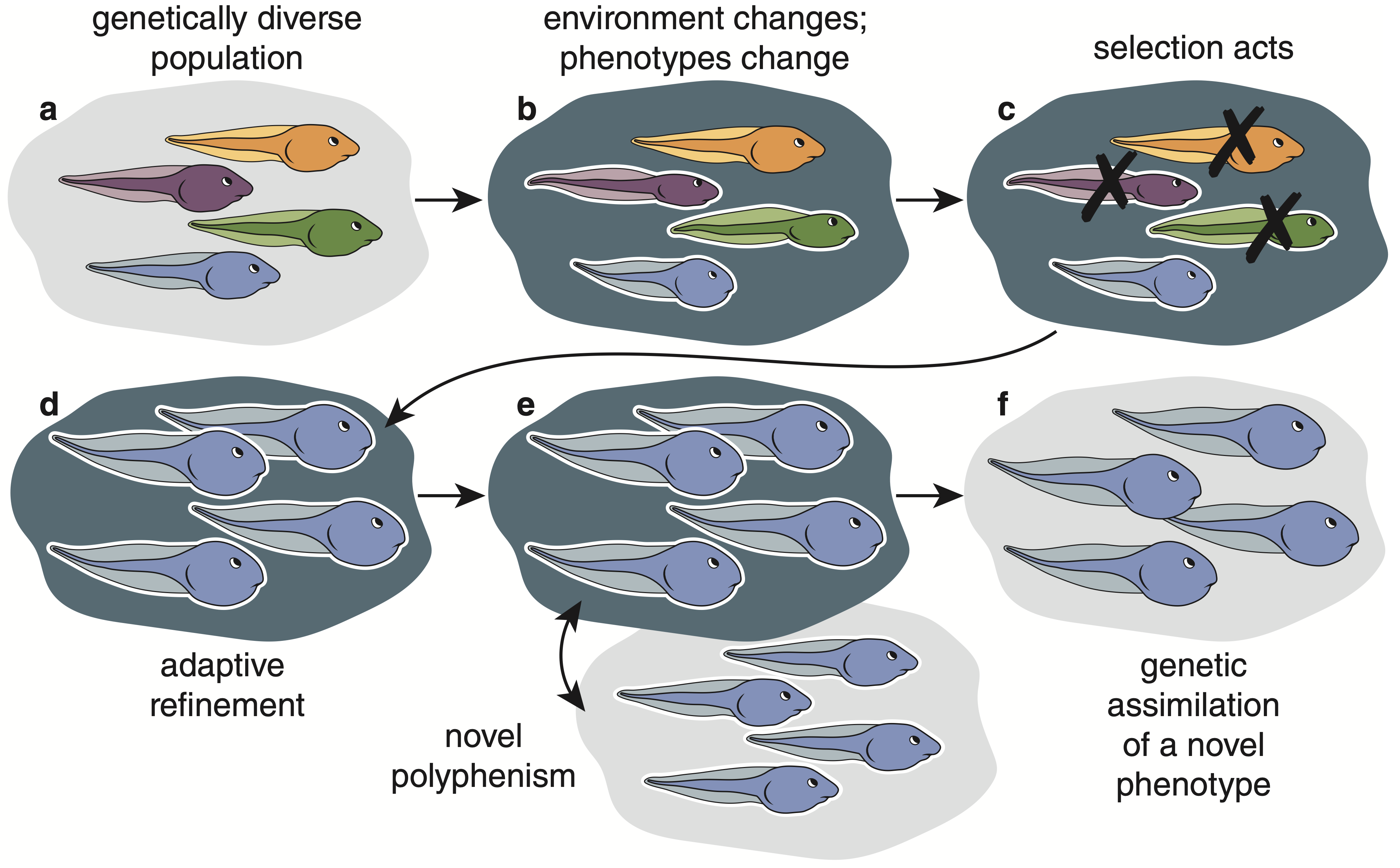
Fig. 1. How plasticity-led evolution promotes the origin of a novel, complex phenotype.
a. In this hypothetical example of plasticity-led evolution, a genetically variable population of tadpoles (represented by different colors, which signify different genotypes)
b. experiences a change in its environment (represented by dark shading) that induces novel phenotypes (represented by white outline), but different genotypes produce different phenotypes (represented by different body shapes).
c. Selection can act on this formerly hidden genetic variation (revealed through plasticity) and disfavor genotypes that produce poorly adapted phenotypes (represented by an ‘X’).
d. This can lead to adaptive refinement of the favored phenotype (represented by the enlargement of the blue tadpole).
e. If individuals produce either this novel phenotype or the ancestral phenotype depending on their environment, then the result is a novel polyphenism.
f. Alternatively, selection might favor the loss of plasticity (i.e., genetic assimilation), resulting in a novel phenotype being expressed regardless of the environment.
Evolution's Wedge: Character Displacement
A major aim of the research in the lab is to explore competition’s role in generating and maintaining biodiversity.
OVERVIEW
Evolutionary biology has long sought to explain the origins of biodiversity. Darwin first proposed a solution to this problem: natural selection. Although natural selection is an evolutionary process, Darwin argued that at its core is an ecological process: competition. According to Darwin, all life forms face recurring competition for scarce resources, and this competition favors individuals that are least like their competitors in resource use and associated traits. Consequently, Darwin held that groups of organisms that compete should become increasingly dissimilar over time, possibly even transforming into separate species. Although these ideas were crucial to Darwin, they remain under appreciated today.
A major aim of the research in my lab is to explore competition’s role in generating and maintaining biodiversity. My colleagues and I have been using spadefoot toads as a model system to understand how competitive interactions can cause populations and species to diverge through an evolutionary process known as character displacement. We have also been investigating how this process can even operate within species and how it might thereby foster the evolution of complex, novel traits. Additionally, we have been studying some of character displacement’s numerous––and far reaching––impacts, such as how it structures ecological communities, promotes the origin of new species, and possibly even underpins certain long-term macroevolutionary patterns.
Recent representative papers:
PDF Levis, N. A., Martin, R. A., O’Donnell, K. A., and Pfennig, D. W. 2017. Intraspecific adaptive radiation: competition, ecological opportunity, and phenotypic diversification within species. Evolution 71: 2496-2509.
PDF Pfennig, K. S., Pfennig, D. W., Porter, C., and Martin, R. A. 2015. Sexual selection’s impacts on ecological specialisation: an experimental test. Proceedings of the Royal Society of London, Series B 282: 20150217.
PDF Bono, L. M., Gensel, C. L., Pfennig, D. W., and Burch, C. L. 2013. Competition and the origins of novelty: experimental evolution of host-range expansion in a virus. Biology Letters 9: 20120616.
BOOK Pfennig, D. W. and Pfennig, K. S. 2012. Evolution’s Wedge: Competition and the Origins of Diversity. University of California Press, Berkeley, CA.
PDF Martin, R. A. and Pfennig, D. W. 2012. Widespread disruptive selection in the wild is associated with intense resource competition. BMC Evolutionary Biology 12: 136.
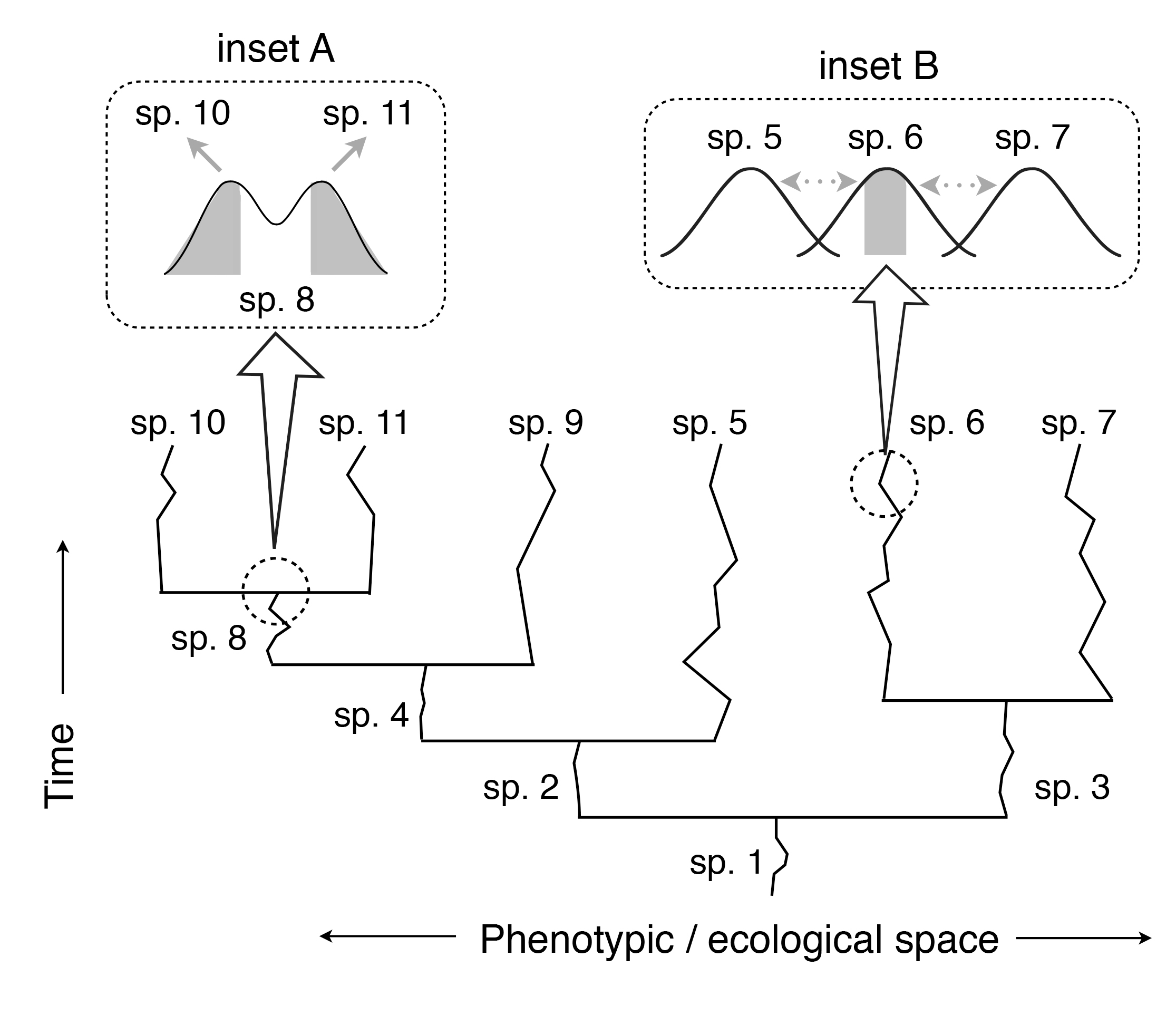
Fig. 2 An illustration depicting how competitively mediated selection––acting both within and between species––may promote the components of an adaptive radiation.
When incipient species (e.g., alternative ecomorphs within a population) compete for resources or for successful reproduction, selection often favors divergence between them as a means of reducing costly competitive interactions. Consequently, speciation may ensue (inset A, where selection favors the shaded portion of species 8’s [sp. 8] phenotypic distribution; here, alternative morphs of species 8 become two new species: species 10 and species 11).
Selection for traits that minimize competitive interactions between species subsequently maintains or enhances phenotypic and ecological differences between them.
As shown in inset B, such selection favors average trait values within each species (again, selection favors the shaded portion of species 6’s phenotypic distribution). Note that, for simplicity, different species are depicted here as occurring along a single phenotypic/ecological axis.
Although actual species will be distributed along multi-dimensional phenotypic/ecological space, closely related species are typically similar phenotypically, ecologically, and reproductively, implying that competitively mediated selection should promote and maintain differences between them.
Life Imitating Life: Mimicry
Although evolutionary biologists have long known about Batesian mimicry, many aspects of its evolution remain unclear.
OVERVIEW
Among nature’s most spectacular adaptations are examples of Batesian mimicry. Batesian mimicry evolves when individuals of a palatable species gain the selective advantage of reduced predation because they resemble a toxic species that predators avoid.
Although evolutionary biologists have long known about Batesian mimicry, many aspects of its evolution remain unclear. We have been investigating such questions as: (1) Why is mimicry often imprecise? (2) What genetic and developmental mechanisms underlie mimicry? (3) What role, if any, does Batesian mimicry play in speciation? and (4) How does Batesian mimicry evolve in the first place?
To address these questions, we have been studying several snake mimicry complexes, one of which is shown above in Fig. 3. The results of some of our field experiments are summarized here.
Recent representative papers:
PDF Akcali, C., Kikuchi, D. W., and Pfennig, D. W. 2018. Coevolutionary arms races in Batesian mimicry? A test of the chase-away hypothesis. Biological Journal of the Linnean Society 124: 668–676.
PDF Akcali, C. and Pfennig, D. W. 2017. Geographic variation in mimetic precision among different species of coral snake mimics. Journal of Evolutionary Biology 30: 1420-1428.
PDF Pfennig, D. W., Akcali, C., and Kikuchi, D. W. 2015. Batesian mimicry promotes pre- and post-mating isolation in a snake mimicry complex. Evolution 69: 1085-1090.
PDF Akcali, C. K. and Pfennig, D. W. 2014. Rapid evolution of mimicry following local model extinction. Biology Letters 10: 20140304.
PDF Kikuchi, D. W. and Pfennig, D. W. 2013. Imperfect mimicry and the limits of natural selection. Quarterly Review of Biology 88: 297-315.
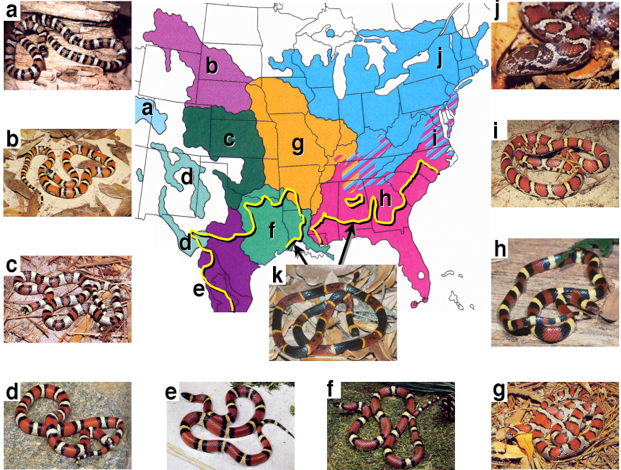
Fig. 3 A snake mimicry complex.
The nonvenomous milk snake Lampropeltis triangulum (panels a-j) is among the most widely distributed snake species, with a geographical range that extends from Canada to Venezuela.
This species also exhibits striking phenotypic variation (variation in the eastern U.S. is shown here).
In areas where it co-occurs with deadly coral snakes, such as the eastern coral snake Micrurus fulvius (panel k), L. triangulum converges on the coral snake’s distinctive warning coloration.
